This Speak to kind is only for Internet site help or Web-site recommendations. If you have thoughts or reviews pertaining to a broadcast document make sure you contact the publishing company.
The program really should be proven to ensure that just the validated parameters for significant functions like sterilization are transferred to standard running processes, and the quality Command unit must assure it.
In addition, this know-how, alone or in combination with cell society, by having an proper detection process, could possibly be made use of as an alternative to one or both equally from the compendial mycoplasma detection techniques soon after suitable validation and settlement from the national regulatory authority.
This website makes use of cookies to help, optimise and analyse site functions, and to supply personalised information and allow you to connect to social media. By clicking "I agree" you consent to the use of cookies for non-vital capabilities as well as linked processing of personal info.
You'll find quite a few resources of contamination during the manufacture of biopharmaceuticals. This incorporates Uncooked supplies, personnel, machines along with the manufacturing surroundings. Regulatory businesses present steering for lowering the risks of introducing bacterial or fungal contamination, such as the utilization of sterilizing methods on raw materials, making sure aseptic circumstances and thru employing GMP.
Evaluation the particular method(s) for your sterilization process picked and the procedures for managing and monitoring the process. Confirm that the process is controlled and monitored.
In the event the company's High quality Method unsuccessful to recognize the process or item nonconformance(s) or get appropriate CAPA, this may be a major CAPA deficiency.
This cookie is about by Cloudflare written content shipping network get more info and is utilized to find out whether it ought to carry on serving “Always On line” till the cookie expires.
Even though the producer might not have detailed documents regarding Objectives 4 and 5 for your contractor's software and personnel, he should have certain the adequacy of these actions with the contractor, by means of activities for instance an audit in the contractor, visits to the contractor, or review of documentation within the contractor. Aim 5 about qualifications with the company's have Q.C. personnel needs to be coated all through your inspection of the maker.
The actions being considered as a Section of program operation for sterility assurance in read more day-to-working day Procedure as well as for the duration of media fill are explained in these 7 measures:
The FDA and other regulatory agencies think about the integrity of laboratory information for being an integral Section of the drug manufacturing process. Deficiencies of out-of-specification (OOS) investigations carry on to become the major cause of warning letters in the pharmaceutical field.
Endotoxin testing will be the process of detecting and quantifying bacterial endotoxins which are existing in pharmaceuticals to make sure the protection and regulatory compliance of the ultimate merchandise satisfies predefined specifications.
The treatment need to Plainly condition exactly where the information will be to be recorded and delineate at what phase testimonials and approvals with the laboratory supervisor and QA are necessary.
Our expert services for materials Evaluation vary from nanomaterials by floor solutions, skinny movies and coatings, to bulk elements.
 Tatyana Ali Then & Now!
Tatyana Ali Then & Now! Keshia Knight Pulliam Then & Now!
Keshia Knight Pulliam Then & Now! Sydney Simpson Then & Now!
Sydney Simpson Then & Now!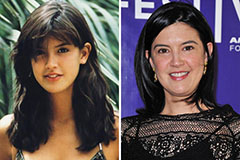 Phoebe Cates Then & Now!
Phoebe Cates Then & Now! Justine Bateman Then & Now!
Justine Bateman Then & Now!